Draft Research Plan for Oyster Gonad Project
This is a draft research plan for a project I could possibly execute winter 2016/2017. I invite any and all feedback. Thanks!
Note: The following plan is specific to O. lurida primarily due to my knowledge of culturing & my NSF proposal focused on this species. However I am interested in opinions regarding possibly shifting to C. gigas in light of the following: I could leverage the already sequenced, aligned & annotated genome; this species does not brood, therefore I could collected/analyze quality of eggs/sperm & fertilization success rate. FYI: Overwintering temperature treatments would need to increase should we opt for C. gigas. In terms of interest, I would happily research either species.
Research Concept: a quick look
A rapidly broadening body of literature indicates that low pH negatively affects many marine invertebrates, much of which has focused on impacts of OA on invertebrate fertilization and larval stages of development, as they are thought to be most sensitive to changing ocean conditions, and therefore the population-level “bottleneck.” However, Kurihara et al. showed that in sea urchins, adults exposed to six months of predicted high levels of CO2 displayed delayed gonad maturation and spawning. No such study has explored the gonadal development and overall larval production response of any bivalve to OA. Larval production, gametogenesis cycling, reproductive energy budgeting, and sex ratios may also respond to acidified waters.
Furthermore, OA is tightly coupled to increasing water temperature, a climate change stressor that must be considered in research that informs predictions of species resiliency. In the case of oysters, temperature is a dominant force influencing distribution, range, and development, and is known to drive the onset and cessation of gametogenesis and spawning. Ostrea lurida, for example, is a protandrous sequential hermaphrodite and begins the spawning season once water temperature reaches approximately 14.5°C to 16°C with male-dominant gonads, then releases the spermatozoa and develops a female-dominant ratio. This alternating process is believed to cease once temperatures drop to below approximately 14.5°C to 16°C, but the influence of abnormally high temperatures is not known. In order to accurately predict future community-level trends of oysters, further research must explore reproductive development and fertility responses to simultaneous stresses of OA and high temperatures.
Perhaps most importantly, the oyster genome has been shown to be highly polymporchic, and Zang et al. (2012) found the C. gigas genome to contain “an extensive set of genes [that respond to] environmental stress.” Paired with the Sweepstakes Reproductive Success hypothesis (e.g. Hedgecock & Pudovkin, 2011) , the oyster genome may contain the adaptive capacity for species resiliency in the face of climate stressors due to differential gene expression, natural selection, or both. Differential gene expression and DNA methylation analysis in the gonads could assist in understanding the underlying mechanisms of oyster adaptability, and could inform hatchery breeding programs to select for OA-resilient oysters, or induce an immune-like response by exposing broodstock to future climate conditions. Similarly, understanding the adaptation capacity (or lack-thereof) of wild populations to low pH and/or high winter temperatures could inform resource managers and restoration groups’ long-term action plans.
In response to these data gaps, this proposal seeks to elucidate the interactive effects of elevated temperature and dissolved CO2 on gonad development, egg/sperm quality, larval production, and differential gene expression & transgenerational selection.
Experimental Plan Overview
Things to sample/measure:
- Broodstock:
- Maturation of gonads over time: level of maturation per Steele & Mulcahy scale; percent area total male & female gametes (via histology).
- Biometric parameters: total weight, shell length, visceral mass weight, condition index (not sure if this is needed)
- Quality of eggs & sperm @ maturation: lipid, protein, and glycogen composition (method?)
- Epigenetics: gene expression & DNA methylation in broodstock gonads over time
- Larval production: Total number produced (if C. gigas: egg/sperm production, fertilization & hatch success rate)
-
Larvae:
- Growth & mortality of larvae over time in 4 treatments
- Gene expression & DNA methylation in larvae over time (Is it possible to get conclusions when comparing gonad-specific DNA in broodstock to the homogenized DNA of a larvae?)
- Water temperature & chemistry: use pH as proxy for DIC and to maintain treatments; process discreet water samples.
Broodstock collection & overwintering:
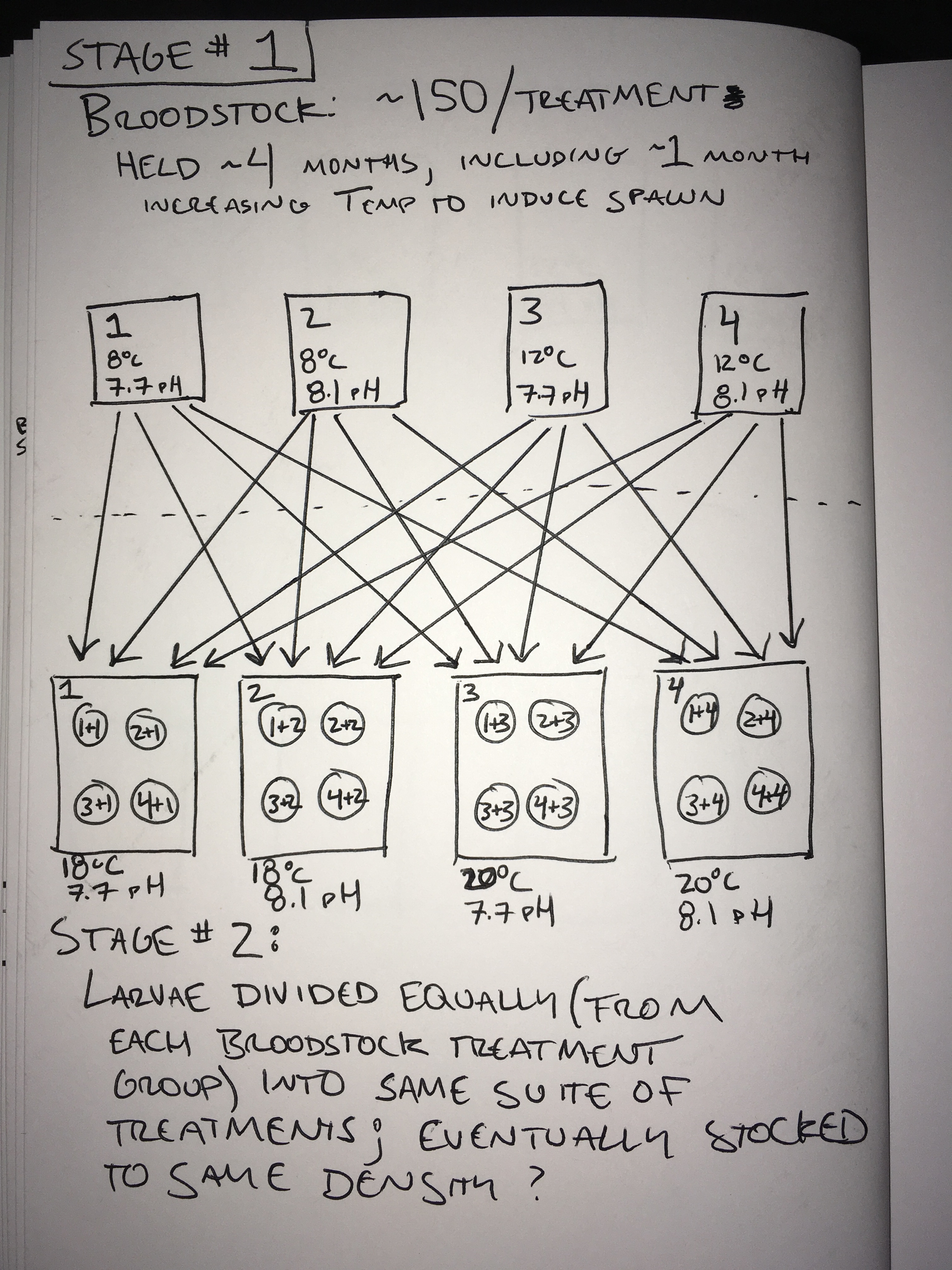
~600 O. lurida individuals will be collected from [where? Dogfish Bay?], and divided into separate flow-through seawater culture tanks. Four treatment regimes, as shown in the below table, will reflect ambient and projected winter water temperatures and dissolved atmospheric CO2. Broodstock will be conditioned in treatments for 3 months; in the 4th month the temperatures will be progressively increased to match the seasonal increase and to induce spawning.
Experimental Treatments:
- Broodstock:
- Treatment #1: pH 8.1 (~400ppm) @ 8°C
- Treatment #2: pH 8.1 (~400ppm) @ 12°C
- Treatment #3: pH 7.7 (~1000pm) @ 8°C
- Treatment #4: pH 7.7 (~1000pm) @ 12°C
- Larvae:
- Treatment #1: pH 8.1 (~400ppm) @ 18°C
- Treatment #2: pH 8.1 (~400ppm) @ 20°C
- Treatment #3: pH 7.7 (~1000pm) @ 18°C
- Treatment #4: pH 7.7 (~1000pm) @ 20°C
Stage 1: Overwintering Conditioning: for up to 4 months (what is the appropriate length of time?) condition broodstock oysters in varying T & pH & induce spawn
- Facility/material needs:
- 4 totes/tubs fitted for flow-through
- 2 aquarium heaters/chillers
- 2 header tanks, 1 equipped with CO2 injector
- 4 temperature probes/data loggers, 1/treatment tote
- Data logging capacity (e.g. Avtech)
- 6 pH probes: 1/CO2-moderated header tank, and 1/treatment tote
- Filtered seawater: 5um sock filters & UV-treated (if possible), consistently available for flow-through system
- Method to aerate/mix needed (method TBD)
- Algae: Consistent algae diet
- Maintenance/labor Needs:
- FSW filter maintenance (weekly at minimum)
- Feeding (daily to every other day, depending on size of header tank)
- Tote cleaning & freshwater rinse (2-3 times/week)
- Monitoring of T & pH and trouble-shooting if needed
- Sampling Needs:
- Discreet water chemistry samples (bi-weekly; 2 bottles per tote)
- Need access to MgCl & chemistry probes for pH, S, T
- Histology samples: bi-weekly, 3 oysters per treatment = 12/week
- Gonad Physiology samples: bi-weekly, 3 oysters per treatment = 12/week
- Epigenetic samples: bi-weekly, 3 oysters per treatment = 12/week
- Microbial community samples (bi-weekly) [[0]I would love to compare bacteria communities between treatments, and/or could we add antibiotics]
- Discreet water chemistry samples (bi-weekly; 2 bottles per tote)
- Total # oysters sampled through conditioning:
- If conditioned 4 months, 36 sampled bi-weekly: 288
- Needed for larval production: ~288
- I would love feedback on the number and timing of each oyster sampling event.
Stage 2: Larval Collection & Rearing: for ~2 weeks, collect, count, sample and rear larvae in varying T & pH
- Facility/Material Needs
- 2 aquarium heaters/chillers
- 2 header tanks, 1 equipped with CO2 injector
- 4 temperature probes/data loggers, 1/treatment tote
- Data logging capacity (e.g. Avtech)
- 6 pH probes: 1/CO2-moderated header tank, and 1/treatment tote
- Filtered seawater: 5um sock filters & UV-treated (if possible), consistently available for flow-through system
- 4 more totes/tubs each fitted for 4 separate inflows (into 4 silos per tote)
- 16 silos with increasing size screen: 60, 100 …
- Method to aerate/mix needed (paddle?)
- Algae: Consistent larval diet free of ciliates
- Screening table, screens sizes (um) 60, 100, 120, 140, 160, 180, 200, 220
- Maintenance/labor Need:
- FSW filter maintenance (weekly at minimum)
- Feeding (daily)
- Screening, silo cleaning & freshwater rinse (every other day)
- Monitoring of T & pH and trouble-shooting if needed
- Sampling Needs:
- Discreet water chemistry samples (every other day; 2 bottles per tote)
- Need access to MgCl & chemistry probes (pH, S, T)
- Larval counts at initial collection (daily)
- Survival counts (every other day, after screening)
- Growth samples/images (every other day, after screening)
- Epigenetic samples (every other day, after screening)
- Microbial community samples (every other day)
- Discreet water chemistry samples (every other day; 2 bottles per tote)
Water Treatment Details
- Temperature: Desired temperatures will be maintained using [Kendra’s aquarium heaters/chillers???] to heat/cool inflowing water as needed; because temperature will need to be increased by increments from overwintering to spawning it would be advantageous to have temperature controllers separate from the hatchery’s system. Beginning in [February? March? April?], all culture tank temperatures will be increased incrementally to 16-18°C to induce spawn. Temperature
- pH: Desired pH levels will be maintained using the Kenneth K. Chew’s ocean acidification experimental CO2 injector system and monitored via Honeywell Durafet pH sensors. dissolved CO2 will remain constant at treatment levels. All groups will be fed identical cocktails of live algae and tanks will be cleaned according to hatchery standards.
Larval collection & rearing details:
- Spawn onset will be monitored daily via screen collection. Once spawning begins larvae will be collected, counted, sampled, and distributed into culture tanks. Daily larvae collection from each broodstock treatment group will be divided into 4 subgroups and distributed into the same 4 treatments. Larvae will be collected, counted & sampled until spawning has ceased (could be unrealistic due to how long spawning can last). Larvae will be stocked into culture tanks until maximum density is reached; any subsequent new larvae produced will not be raised (this is due to space constraints; however if possible I’d like to avoid throwing out the larvae that are spawned late in the game).