Terminating 3-month Oly temp/food treatment
It’s been 3~ months since Olys went into their temperature / feeding treatments. I’ve been collecting gonad samples for histology every 2-3 weeks. Today I did a 6th sampling from treatments and “wild” (aka directly from Mud Bay) and moved animals into their spawning buckets. Details …
Sampled gonad tissue for 2 things today: 1) histology and 2) lipid & glycogen content
To accomplish this, I shucked the oyster, removed the gill tissue as usual by grabbing with gripped tweeers and tearing back the tissue (usually removes cleaniy, without removing any gonad). Then, I used dissecting scissors to bisect the whole visceral mass into two sections. The cut was made perpendicular to and midway along the anterior/posterior axis, as shown below. The anterior section was fixed for histology, and the posterior pulled from from the adductor, then placed into a labeled 2mL tube for glycogen/lipid analysis. Oysters were sampled in batches of 5 (from each replicate), and immediately walked to the -80 in Rick’s building to freeze. The posterior section was selected for lipid/glycogen analysis, as it was free of gill or palp tissue (not always the case in the anterior section). Mantle tissue on dorsal/ventral planes of visceral mass was not intentionally removed. Frozen gonad samples were labeled with their designated histology sample number (from 241-290; there are no frozen gonad tissue samples #1-239).
Bottom prong of tweezer indicating where transverse cut will be made:
Video of the “incision”
Post-cut.
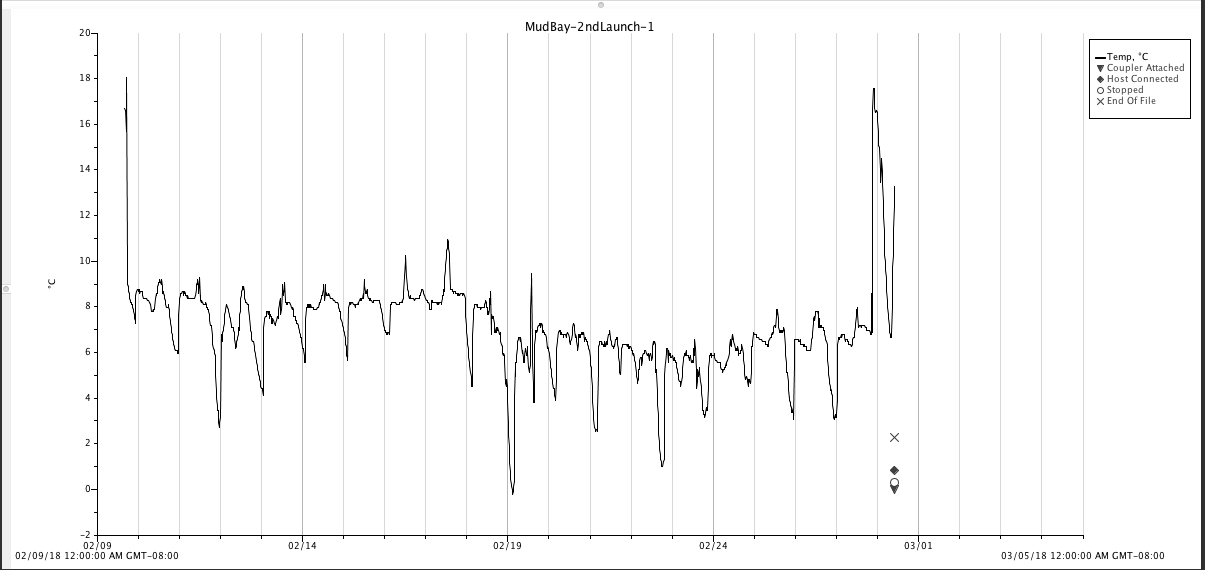
Final Mud Bay sampling, retrieved HOBO loggers
As usual, I collected 10 oysters from Mud Bay during low tide (today, ~8:30pm), and took back to the hatchery for sampling. Tonight I also retrieved the 2 HOBO data loggers. One HOBO was installed at a shallow depth, accessible around ~2’, close to where I harvested oysters throughout the winter (labeled “Mud Bay Shallow” & “Mud Bay Shallow - redeployment). The other was installed at a low tidal elevation near the main channel, away from my sampling site (labeled “Mud Bay Deep”).
Screen-shot of the Mud Bay “Deep” data

Screen-shot of the Mud Bay “Shallow” data
It’s in 2 plots, as I deployed twice over concern of battery/space. Note the different date ranges along the x-axes

Moved animals to spawning buckets
Decided on 4 spawning buckets/treatment. This results in ~45-50 animals per spawning bucket and I was able to simply split the 4 bags in each treatment rep. This makes it easy to keep track of replicates from the treatment to the spawning stage. For example, in treatment A1 there were 4 backs (#1, 15, 5, 15). These were split into 2 spawning buckets (#1 + 16, #5 + 15).
First, I counted the # oysters in each treatment bag, and measured volume displacement. Volume displacement was measured using a 1000mL graduate cylinder filled to ~500mL, then I removed the oysters from their bag and trasferred to the grad. cylinder, and measured final volume. I selected 2-bag spawning “pairs” using volume displacement to acheive consistent volume across reps.
In summary: For each treatment (A, B, C, D) I have 4 replicate spawning buckets: 2 from treatment replicate 1 (e.g. A1-1, A1-2 … D1-1, D1-2), and 2 from treatment replicate 2 (e.g. A2-1, A2-2 … D2-1, D2-2). All buckets were installed with air stones. Avtech loggers were placed back in their respective treatments.
Terminated experiment, adjusted things
Turned chiller in low temp up 1degC. Plan is to increase temperature on low temp by 1degC per day unti it reaches 10degC, then all buckets will be increased by 1degC/day until 18degC is acheieved on ~3/10. Ryan said he would turn temp up for me.
Turned up low food algae dosing rate to match high fodd rate (175 on peristaltic dosing pump).
Next steps & To-Do’s
I expect to get larvae during the week of 3/26
Will visit Manchester on Tuesdays (and maybe Sundays too) to clean broodstock, check mortality.
Inventory the # of 1L silos, estimate now many I will need, and build more.
Get OA system back up and running.
Plan OA stress trials for larvae

